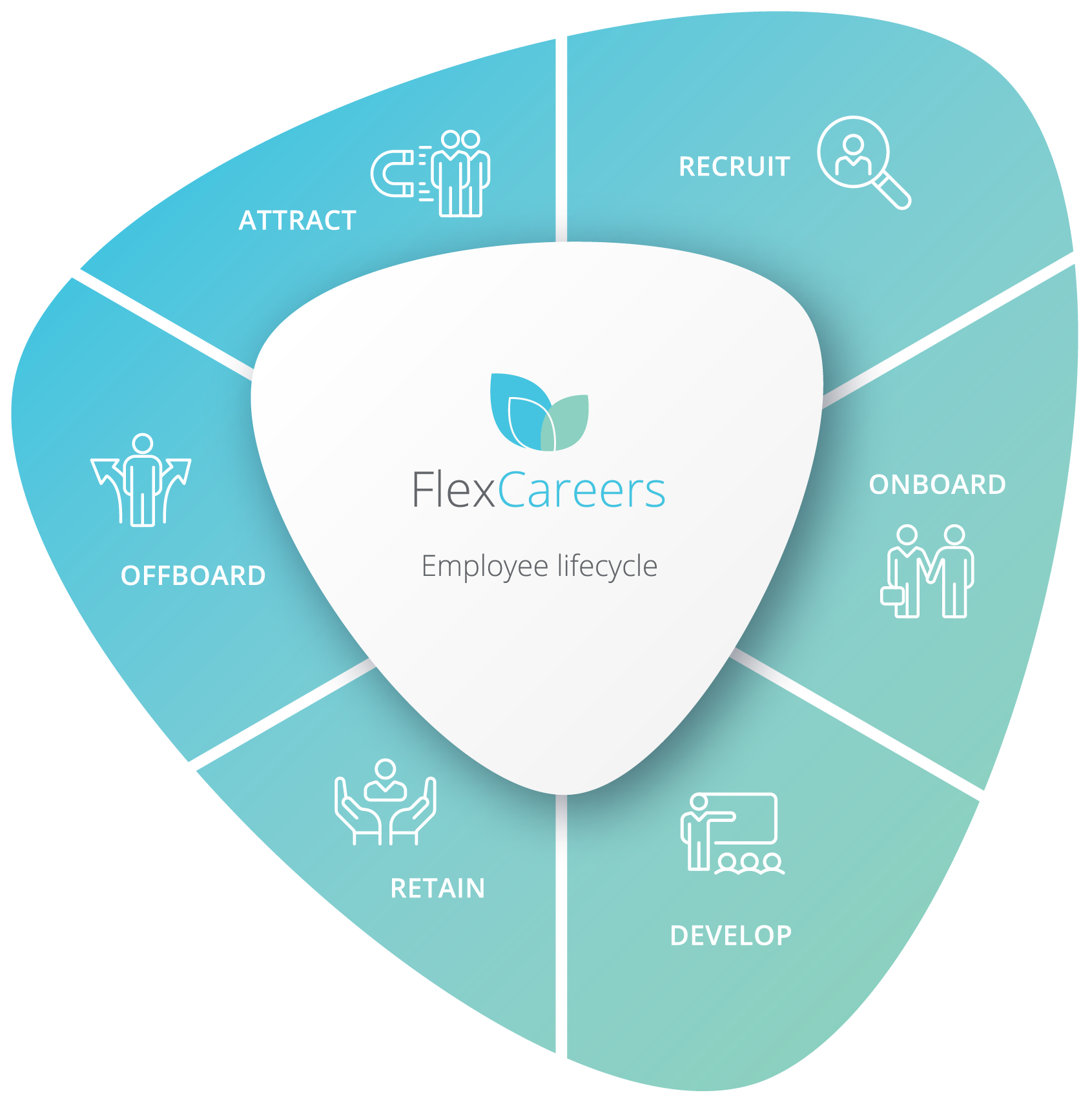
Lifecycle Solutions
Attract
Drive your employer brand awareness & engage diverse talent through a defined authentic EVP and a standout flexibility offering.
Recruit
Find & source diverse quality candidates through engaged talent pipelines and the leading jobs marketplace for flexible work opportunities.
Onboard
Deliver on your EVP promise, empower with flexibility and provide a great experience to engage your new starters & set them up for success.
Develop
Empower your people and support their development with flexibility options to balance their work life family & career goals.
Retain
Engage your people and increase productivity with a leading flexible work experience and programs for parental & carer’s leave.
Offboard
Support your people as they transition out take a career break or go on parental leave and ultimately become ambassadors for your brand.
For job seekers __________________________________________________________
Live your best life with a career that works for you


For job seekers __________________________________________________________
Live your best life with a career that works for you

For employers____________________________________________________________________________
Harness flexibility
to create an engaged and productive workforce




































